Introduction
Currently, venetoclax is the therapeutic choice for many patients with chronic lymphocytic leukemia progressed on ibrutinib. The optimal drug combinations with venetoclax, the goals and timing of therapy, as well as predictors of response are not yet clear. Only a few clinical trials have included patients with progression on ibrutinib (Kater AP, et.al., J Clin Oncol 2019). Approach to ibrutinib discontinuation and addition of antibodies to CD20 are still a matter of debates.
Objectives
The aim of this multicenter study was to retrospectively compare outcomes in patients who received venetoclax either alone or in combination with monoclonal antibodies to CD20, as well as outcome of patients who continued ibrutinib after switching to venetoclax.
Methods
The study included 122 patients with disease progression on ibrutinib according to iwCLL2018 criteria. The data were collected by 27 centers from 25 regions of Russia. Fifty four patients (44%) received venetoclax without monoclonal antibodies, 46 (38%) in combination with obinutuzumab, 22 (18%) with rituximab. Nineteen patients stopped ibrutinib before venetoclax with median interval between treatments of 10 days (range 0 - 91 days). All other patients received venetoclax concomitantly with ibrutinib for some time after progression. Patients who stopped ibrutinib less than 3 months from the date of onset of venetoclax were considered to have discontinued ibrutinib.
Results
The median age was 63 years (range 30-82), 76 patients were males (62%). Median progression free survival (PFS) for all patients was 26.5 months, overall survival (OS) was 35.2 months. Fifteen patients (12%) had Richter transformation before venetoclax. Overall survival of these patients was significantly worse when compared to other patients, with hazard ratio (HR) 3.46 95% CI 1,42 - 8,48 (p<0.0001). Twenty two patients stopped therapy before the event of death or progression. Reasons included toxicity (N=10), three consecutive confirmation of MRD-negative remission 3 months apart (N=11), and allogeneic stem cell transplantation (N=1).
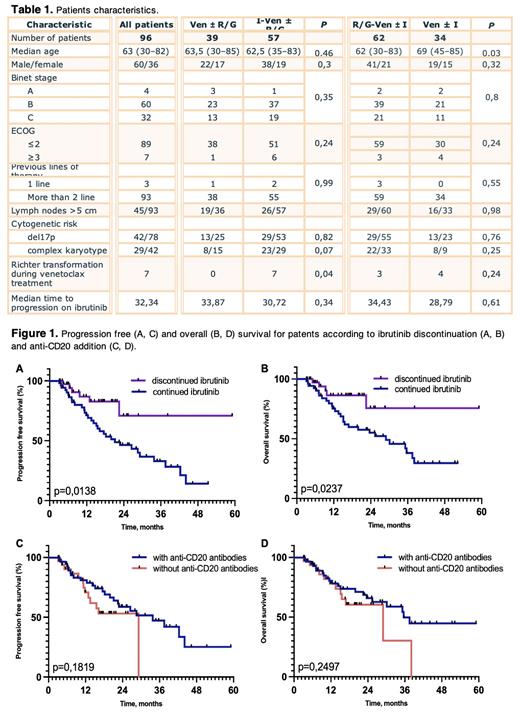
Patients who died or had progression within 3 months after the onset of venetoclax, as well as patients with Richter syndrome diagnosed before the onset of venetoclax were excluded from survival analysis. Table 1 presents patients' characteristics. On pre-treatment, poor outcome was significantly predicted by time to progression on ibrutinib <24 months (PFS: HR 0.53, 95% CI 0.27 - 1.06, p=0,04; OS: HR 0.45, 95% CI 0,22 - 0,93, p=0,01) and ECOG status > 2 (PFS: HR 0.37, 95% CI 0,1 - 1,35, p=0,007; OS: HR 0.32, 95% CI 0,08 - 1.27, p=0,002). There were no differences in baseline characteristics in patients who continued and discontinued ibrutinib, although in the former group more patients have developed Richter syndrome during treatment. Ibrutinib discontinuation was associated with better outcome (PFS: HR 3.72, 95% CI 1.95 - 7.09; OS: HR 3.93, 95% CI 1.96 - 7.88, Figure 1A, B). In total 15 deaths occurred before progression (COVID-19 - 11 patients, secondary cancers - 2, sudden death - 1, pneumonia - 1), and the majority (N = 13) in the group of patients who continued ibrutinib.
The group of patients who received antibodies to CD20 was younger (63 versus 69 years, p=0.03), while other baseline parameters were similar. No significant differences were found in either PFS (HR 1.38, 95% CI 0.69 - 2.7) or OS (HR 1.36, 95% CI 0.67 - 2.8, Figure 1C, D). Subgroup analysis of patients who discontinued ibrutinib showed that patients who received venetoclax with monoclonal antibodies had better PFS compared to those on venetoclax alone (HR 6.32, 95% CI 1.43 - 27.8, p=0.045), while OS in the two subgroups did not differ.
Conclusion
Our data do not support the hypothesis that continuing ibrutinib therapy beyond 2 - 3 months in the context of progression on ibrutinib benefits patients. As evident from increase in PFS, it seems that patients who had discontinued ibrutinib may benefit from adding CD20 antibodies to venetoclax.
Disclosures
Maschan:Miltenyi Biotec: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal